
Ultrasound and clinical characteristics of patients with COVID-19 pneumonia: a cross-sectional study
Highlight box
Key findings
• Severe/critical COVID-19 pneumonia demonstrated higher BUN, albumin, CK-MB, NT-proBNP, CRP, and LDH level than moderate cases. LUS score, which is simple and handily accessible, could help in the evaluation of the severity and prognosis of COVID-19 pneumonia.
What is known and what is new?
• LUS was effectively used to diagnose acute respiratory distress syndrome, monitor the response to treatment, and detect bacterial superinfection, as well as COVID-19 pneumonia.
• There is a paucity of evidence on assessing the association between LUS characteristics and the severity of COVID-19.
What is the implication, and what should change now?
• Studying the clinical and ultrasound characteristics of COVID-19 help understand the features of critical conditions and promote evaluation and therapeutical decisions.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by infection of severe acute respiratory syndrome coronavirus 2 (1,2). Early diagnosis and timely intervention can prevent the disease from developing into a severe/critical state (1). The diagnosis of COVID-19 relied on real-time fluorescence quantitative reverse transcription polymerase chain reaction (RT-PCR) to detect viral nucleic acid and chest computed tomography (CT) scan (3,4). However, disinfection after using the CT machine will delay the care of other patients who require CT examination (5). Lung ultrasound (LUS) examination is significant in the early screening of suspected cases because it can be performed without conveying the patient from the intensive care unit (ICU) or ward (6,7). LUS reduces the risk of viral disease among medical staff and gets an immediate result (6,7). At the same time, LUS, as a useful tool to assess lung pathophysiology, also has practical value in evaluating changes in lung ventilation (8,9). However, there is little research reported on the impact of the detailed characteristics of LUS on the severity of COVID-19. This study aimed to demonstrate the clinical and bedside LUS features at hospital admission related to the severity and survival of patients with COVID-19 pneumonia. We present the following article in accordance with the STROBE reporting checklist (available at https://aoi.amegroups.com/article/view/10.21037/aoi-22-4/rc).
Methods
Data collection
It was a single-center, retrospective, cross-sectional study. Between 22 January 2020 and 3 March 2020, consecutive patients confirmed COVID-19 pneumonia established by the World Health Organization interim guidance (10) were included in the First People’s Hospital of Jingzhou, China. COVID-19 infection was diagnosed via RT-PCR using the throat-swab specimens. Patients with missing data of LUS data or major laboratory tests were excluded. The major laboratory test consisted of a complete blood count, blood urea nitrogen (BUN), albumin, serum creatinine, prothrombin time, activated partial thromboplastin time (APTT), D-dimer, lactate dehydrogenase (LDH), immunoglobulin M (IgM) antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), immunoglobulin G (IgG) antibodies against SARS-CoV-2, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First People’s Hospital of Jingzhou and Guangdong Provincial People’s Hospital (No. 2020-056), and written informed consent was waived.
Clinical and ultrasonic evaluation
According to the guideline on the management of COVID-19 (6th edition) issued by the National Health Commission (11) and the Proposal for use of LUS (12,13), the severity was classified (Table 1), and point of care LUS was scanned systematically from the front to the backside of each hemithorax with the patient in the sitting position (14). Patients were admitted to ICU or isolated wards at the discretion of the physicians according to the severity of the disease. Baseline demographic and clinical information, ultrasound features, and survival status were recorded. Disease scoring systems were calculated by the physicians or nurses, including acute physiology and chronic health evaluation II (APACHE II) score (13), the sequential organ failure assessment (SOFA) score (15), and the Glasgow Coma Scale (GCS). The scores represent the worst scores during the first 24 hours after ICU admission or hospitalization.
Table 1
| Classification | Condition |
|---|---|
| Severe case | Dyspnea with a respiratory rate of ≥30 times/minute |
| Saturation ≤93% | |
| PaO2/FiO2 ≤300 mmHg | |
| Critical case | Respiratory failure requiring mechanical ventilation |
| Shock | |
| Co-existing multiple organ failure requiring close monitoring in the ICU |
Severe/critical case was diagnosed if one of the corresponding conditions was present; COVID-19, coronavirus disease 2019; PaO2, arterial partial pressure of oxygen; FiO2, fractional inspiratory oxygen concentration; ICU, intensive care unit.
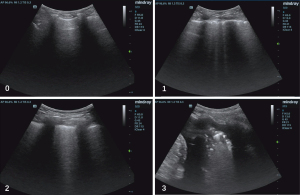
LUS was performed using a 2- to 4-MHz convex probe by trained physicians. Five regions of interest on each side were investigated according to the BLUE-Protocol (16), including the upper and lower BLUE-point in the anterior/posterior zone and posterolateral alveolar and/or pleural syndrome (PLAPS) point. Points were allocated according to the worst ultrasound pattern observed: (I) normal aeration (0 points): horizontal A-lines (or no more than two B-lines); (II) moderate loss of aeration (1 point): multiple B lines (either regularly spaced, or irregularly and even coalescent, but only visible in a limited area of the intercostal space); (III) severe loss of aeration (2 points): multiple coalescent B lines in prevalent areas of the intercostal spaces and observed in one or several intercostal spaces; and (IV) complete loss of aeration (3 points): lung consolidation with or without air bronchograms (Figure 1). The abnormal findings in each LUS scan were summed up with a minimum score of zero and a maximum score of 30 (17,18).
Statistical analysis
Data were analyzed using SPSS 21.0 for Windows (IBM Corp. Armonk, NY, USA). The continuous values were presented as the means ± standard deviation or median (quartiles) and were compared using independent t-tests or the Mann-Whitney test, according to their distribution. The categorical variables were presented as counts (percentages) and were compared using the chi-square test or Fisher exact test. A two-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Among 146 patients, a total of 36 patients with ultrasound data were finally analyzed. Demographics, clinical, and laboratory findings were reported in Table 2. Twenty-nine (80.6%) patients were severe/critical cases, and 7 (19.4%) were moderate cases. The median age was 65.1 years, ranging from 21 to 89 years. Compared with moderate patients, severe patients showed lower levels of creatine kinase (CK) (22.0 vs. 60.0 U/L, P=0.018), but a higher level of BUN (5.3 vs. 3.8 mmol/L, P=0.036), albumin (43.1 vs. 36.4 U/L, P=0.001), creatine kinase-MB (CK-MB) (9.0 vs. 7.0 U/L, P=0.011), N-terminal pro-brain natriuretic peptide (NT-proBNP) (68.7 vs. 8.2 pg/mL, P=0.006), C-reactive protein (CRP) (17.6 vs. 1.7 mg/L, P=0.008), LDH (198.0 vs. 139.0 U/L, P=0.026), and higher SOFA scores (2.0 vs. 0.0 points, P=0.001). There was no significant difference in age, gender, low IgM, IgG, ALT, AST, TBIL, DBIL, white blood cell (WBC), procalcitonin (PCT), and monocyte count between severe and moderate patients (P>0.05).
Table 2
| Parameters | Total (n=36) | Severe/critical group (n=29) | Moderate group (n=7) | P value |
|---|---|---|---|---|
| Age (years) | 64.3±15.2 | 67.0±14.0 | 54.0±19.0 | 0.138 |
| Gender | ||||
| Male | 20 (55.6) | 16 (55.2) | 4 (57.1) | 0.631 |
| Female | 16 (44.4) | 13 (44.8) | 3 (42.9) | |
| Oxygen device | 0.011 | |||
| Without oxygenation therapy | 3 (8.3) | 0 (0.0) | 3 (42.9) | |
| Nasal oxygen | 27 (75.0) | 23 (79.3) | 4 (57.1) | |
| Mask oxygen | 1 (2.8) | 1 (3.4) | 0 (0.0) | |
| Mechanical ventilation | 5 (13.9) | 5 (17.2) | 0 (0.0) | |
| APACHE II score | 6.0 (4.3–8.0) | 6.0 (5.0–8.0) | 4.0 (0.0–6.0) | 0.440 |
| SOFA score | 1.0 (0.0–2.8) | 2.0 (1.0–4.0) | 0.0 (0.0–0.0) | 0.001 |
| GCS | 15.0 (0.0–2.8) | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) | 0.345 |
| WBC (×109/L) | 6.1 (5.0–8.0) | 6.1 (5.0–9.6) | 6.0 (4.4–7.1) | 0.584 |
| Lymphocytes (×109/L) | 1.4±0.6 | 1.3±0.6 | 1.8±0.4 | 0.050 |
| Monocyte count (×109/L) | 0.4 (0.3–0.5) | 0.4 (0.3±0.5) | 0.3 (0.2±0.9) | 0.531 |
| PCT (ng/mL) | 1.0 (0.3–5.2) | 1.2 (0.3–6.4) | 0.59 (0.3–3.4) | 0.584 |
| CRP (mg/L) | 6.4 (2.1–4.3) | 17.6 (2.6–72.8) | 1.7 (0.5–3.8) | 0.008 |
| BUN (mmol/L) | 5.1 (3.9–6.2) | 5.3 (4.2–6.5) | 3.8 (3.0–5.0) | 0.036 |
| Creatinine clearance (μmol/L) | 58.0 (45.7–74.8) | 61.1±22.5 | 65.8±17.3 | 0.606 |
| ALT (U/L) | 27.0 (14.0–36.8) | 27.0 (14.0–36.5) | 23.0 (12.0–37.0) | 0.938 |
| AST (U/L) | 26.0 (22.0–33.0) | 25.0 (22.0–33.0) | 27.0 (18.0–29.0) | 0.696 |
| TBIL (μmol/L) | 10.5 (8.9–17.4) | 10.6 (8.2–18.3) | 9.6 (9.4–13.9) | 0.584 |
| DBIL (μmol/L) | 3.7 (2.4–6.7) | 3.8 (2.5–8.0) | 2.8 (2.3–5.5) | 0.505 |
| Albumin (g/L) | 37.0 (33.0–40.9) | 43.1 (42.1–43.7) | 36.4 (32.4–39.5) | 0.001 |
| Prothrombin time (s) | 10.5 (10.0–11.5) | 10.5 (9.6–11.6) | 10.3 (10.0–11.5) | 0.845 |
| APTT (s) | 25.5 (22.8–31.1) | 27.2±6.6 | 25.6±4.4 | 0.543 |
| D-dimer (mg/L) | 3.7 (0.5–6.0) | 3.4 (0.7–6.1) | 2.1 (0.2–6.0) | 0.131 |
| Troponin T (U/L) | 18.7 (7.4–37.2) | 13.3 (6.0–34.1) | 29.0 (24.7–104.5) | 0.086 |
| CK (U/L) | 39.0 (18.3–59.8) | 22.0 (15.0–53.0) | 60.0 (48.0–76.0) | 0.018 |
| CK-MB (U/L) | 9.0 (7.0–11.8) | 9.0 (7.5–12.0) | 7.0 (6.0–8.0) | 0.011 |
| NT-proBNP (pg/mL) | 60.1 (11.4–123.3) | 68.7 (27.9–128.8) | 8.2 (6.8–11.2) | 0.006 |
| LDH (U/L) | 189.0 (162.3–238.0) | 198.0 (171.5–250.0) | 139.0 (124.0–189.0) | 0.026 |
| IgM* (g/L) | 25.9 (14.2–105.7) | 25.6 (12.4–107.9) | 49.4 (21.3–108.9) | 0.554 |
| IgG* (g/L) | 174.4 (137.5–207.5) | 174.3 (139.9–204.3) | 57.9 (116.6–229.0) | 0.738 |
The continuous values were presented as the means ± standard deviation or median (quartiles) according to their distribution; the categorical variables were presented as counts (percentages). *, antibodies against SARS-CoV-2. APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; GCS, Glasgow Coma Scale; WBC, white blood cell; PCT, procalcitonin; CRP, C-reactive protein; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; APTT, activated partial thromboplastin time; CK, creatine kinase; CK-MB, creatine kinase-MB; NT-proBNP, N-terminal pro-brain natriuretic peptide; LDH, lactate dehydrogenase; IgM, immunoglobulin M; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ultrasound findings in the severe/critical group and moderate group
Ultrasound characteristics were described in Table 3. Echocardiography and LUS examination for 36 patients were performed after admission. No significant difference was found between the severe group and the moderate group, including the left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE), and mitral annular plane systolic excursion (MAPSE) (P>0.05). The inferior vena cava (IVC) value on inhalation (10.6 vs. 5.1 mm, P<0.001) in severe/critical patients was higher than those of the moderate group. The LUS score in the severe/critical group was also higher than those in the moderate group (9.0 vs. 2.0 points, P<0.001). Each acquisition point of LUS was scored. The LUS score of severe/critical group patients in the posterior blue point (3.0 vs. 1.0 points, P=0.040) and diaphragmatic point (4.0 vs. 0.0 points, P=0.001) were significantly higher than those of the moderate group. The incidences of the alveolar-interstitial syndrome (48.3% vs. 0.0%, P=0.029), lung consolidations (96.6% vs. 57.1%, P=0.003), and lung shred signs (89.7% vs. 42.9%, P=0.009) in severe/critical patients were higher than those of the moderate patients. The consolidated locations were different between the two groups.
Table 3
| Parameters | Total (n=36) | Severe/critical group (n=29) | Moderate group (n=7) | P value |
|---|---|---|---|---|
| LUS findings | ||||
| LUS score | 8.0 (4.0–13.0) | 9.0 (6.0–15.0) | 2.0 (0.0–4.0) | <0.001 |
| Upper blue point score | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.410 |
| Lower blue point score | 0.0 (0.0–1.0) | 0.0 (0.0–3.0) | 0.0 (0.0–0.0) | 0.178 |
| Diaphragmatic point score | 3.0 (1.0–6.0) | 4.0 (3.0–6.0) | 0.0 (0.0–3.0) | 0.001 |
| Posterior blue points score | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 1.0 (0.0–3.0) | 0.04 |
| Alveolar-interstitial syndrome | 14 (38.9) | 14 (48.3) | 0 (0.0) | 0.029 |
| Pleural effusion | 10 (27.8) | 10 (34.5) | 0 (0.0) | 0.079 |
| Lung consolidations | 32 (88.9) | 28 (96.6) | 3 (42.9) | 0.003 |
| Shred signs | 29 (80.6) | 26 (89.7) | 3 (42.9) | 0.009 |
| Tissue-like sign | 4 (11.1) | 4 (13.8) | 0 (0.0) | 0.566 |
| Where the lung consolidations appeared | 0.003 | |||
| No lung consolidations | 5 (13.9) | 1 (3.4) | 4 (57.1) | |
| Upper blue point | 2 (5.6) | 2 (6.9) | 0 (0.0) | |
| Lower blue point | 5 (13.9) | 5 (17.2) | 0 (0.0) | |
| Diaphragmatic point | 2 (5.6) | 2 (6.9) | 0 (0.0) | |
| Posterior blue points | 16 (44.4) | 14 (48.3) | 2 (28.6) | |
| PLAPS points | 9 (25.0) | 8 (27.6) | 1 (14.3) | |
| Echocardiography findings | ||||
| LVEF (%) | 55.0±8.0 | 54.0±8.0 | 56.0±3.0 | 0.492 |
| IVCe (mm) | 16.2±3.8 | 16.8±3.6 | 13.8±4.2 | 0.064 |
| IVCi (mm) | 9.4±5.0 | 10.6±5.0 | 5.1±1.0 | <0.001 |
| cIVC | 0.4±0.2 | 0.4±0.20 | 0.6±0.1 | <0.001 |
| TAPSE (mm) | 20.7±4.3 | 20.9±4.2 | 19.6±4.9 | 0.496 |
| MAPSE (mm) | 13.0±3.5 | 12.9±3.7 | 13.8±3.0 | 0.504 |
The continuous values were presented as the means ± standard deviation or median (quartiles) according to their distribution; the categorical variables were presented as counts (percentages). COVID-19, coronavirus disease 2019; LUS, lung ultrasound; PLAPS, posterolateral alveolar and/or pleural syndrome; LVEF, left ventricular ejection fraction; IVCe, inferior vena cava maximal diameter during expiration; IVCi, inferior vena cava maximal diameter during inhalation; cIVC, collapsibility index of inferior vena cava; TAPSE, tricuspid annular plane systolic excursion; MAPSE, mitral annular plane systolic excursion.
Clinical characteristics of survivor and non-survivor group
As shown in Table 4, 31 patients recovered and were discharged, while 5 patients died in the hospital. The lethal cases were all diagnosed with severe (2 cases) and critical (3 cases) illnesses. The survivors and non-survivors had no significant differences in gender or age (P>0.05). The APACHE II (25.0 vs. 6.0 points, P<0.001) and SOFA scores (11.0 vs. 1.0 points, P<0.001) of non-survivors were significantly higher than survivors. WBC (10.6×109/L vs. 6.0×109/L, P=0.030), BUN (9.4 vs. 5.0 mmol/L, P=0.020), TBIL (28.0 vs. 10.2 µmol/L, P=0.019), DBIL (14.0 vs. 3.2 µmol/L, P=0.012), and CRP levels (86.2 vs. 4.6 mg/L, P<0.001) in the non-survivors’ group were higher than those in the survivors’ group. Compared with the survivors, the non-survivors had a lower lymphocyte count (0.8 vs. 1.5×109/L, P=0.020) and albumin (31.1 vs. 38.9 g/L, P=0.007) on admission. No significant differences in IgM, IgG, AST, PCT, APTT, and monocyte count were found between the two groups (P>0.05).
Table 4
| Parameters | Survivor (n=31) | Non-survivor (n=5) | P value |
|---|---|---|---|
| Age (years) | 64.0±16.0 | 63.0±11.0 | 0.890 |
| Gender | |||
| Male | 17 (54.8) | 3 (60.0) | 0.610 |
| Female | 14 (45.2) | 2 (40.0) | |
| Oxygen device | 0.030 | ||
| Without oxygenation therapy | 3 (9.7) | 0 (0.0) | |
| Nasal cannula | 25 (80.6) | 2 (40.0) | |
| Mask oxygen | 1 (3.2) | 0 (0.0) | |
| Mechanical ventilation | 2 (6.5) | 3 (60.0) | |
| APACHE II score | 6.0±3.0 | 25.0±16.0 | <0.001 |
| SOFA score | 1.0 (0.0–2.0) | 11.0 (5.0–12.5) | <0.001 |
| GCS | 15.0 (15.0–15.0) | 3.0 (3.0–15.0) | 0.059 |
| WBC (×109/L) | 6.0 (4.4–7.1) | 10.6 (6.6–21.0) | 0.030 |
| Lymphocytes (×109/L) | 1.5±0.6 | 0.8±0.5 | 0.020 |
| Monocyte count (×109/L) | 0.4 (0.3–0.5) | 0.28 (0.16–0.88) | 0.450 |
| PCT (ng/mL) | 0.7 (0.3–6.3) | 1.2 (0.8–2.4) | 0.690 |
| CRP (mg/L) | 4.6 (1.7–20.0) | 86.2 (72.8–183.9) | <0.001 |
| BUN (mmol/L) | 5.0 (3.7–5.7) | 9.4 (5.4–10.2) | 0.020 |
| Creatinine clearance (μmol/L) | 60.5 (46.7–74.8) | 53.0 (27.1–69.0) | 0.496 |
| Albumin (g/L) | 38.9±4.8 | 31.1±6.8 | 0.007 |
| ALT (U/L) | 27.0 (14.0–37.0) | 22.0 (12.0–34.0) | 0.861 |
| AST (U/L) | 25.0 (21.0–33.0) | 34.0 (22.5–48.5) | 0.295 |
| TBIL (μmol/L) | 10.2 (8.8–13.9) | 28.0 (14.5–37.2) | 0.019 |
| DBIL (μmol/L) | 3.2 (2.1–5.5) | 14.0 (8.0–30.2) | 0.012 |
| Prothrombin time (s) | 10.4 (10.0–11.5) | 11.2 (9.7–11.9) | 0.825 |
| APTT (s) | 26.7±6.5 | 28.2±4.3 | 0.629 |
| D-dimer (mg/L) | 2.0 (0.45–6.01) | 3.6 (0.4–7.2) | 0.967 |
| CK (U/L) | 36.0 (19.0–60.0) | 42.0 (12.0–242.0) | 0.910 |
| CK-MB (U/L) | 8.9 (7.0–11.0) | 17.0 (8.0–26.0) | 0.066 |
| Troponin T (U/L) | 17.3 (7.5–34.6) | 24.0 (1.8–92.5) | 0.760 |
| NT-proBNP (pg/mL) | 55.0 (10.6–105.4) | 131.5 (83.7–1,699.9) | 0.056 |
| LDH (U/L) | 186.0 (160.2–208.0) | 267.0 (183.5–531.1) | 0.226 |
| IgM* (g/L) | 30.2 (16.8–120.0) | 21.7 (4.4–59.9) | 0.361 |
| IgG* (g/L) | 175.1 (144.6–219.8) | 156.6 (80.9–206.7) | 0.718 |
The continuous values were presented as the means ± standard deviation or median (quartiles) according to their distribution; the categorical variables were presented as counts (percentages). *, antibodies against SARS-CoV-2. COVID-19, coronavirus disease 2019; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; GCS, Glasgow Coma Scale; WBC, white blood cell; PCT, procalcitonin; CRP, C-reactive protein; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; APTT, activated partial thromboplastin time; CK, creatine kinase; CK-MB, creatine kinase-MB; NT-proBNP, N-terminal pro-brain natriuretic peptide; LDH, lactate dehydrogenase; IgM, immunoglobulin M; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ultrasound findings are summarized in Table 5. The LUS score of the mortality was significantly higher than that of the survival group (17.0 vs. 7.0 points, P=0.003). We scored each acquisition point of LUS. The results showed that the scores of the upper blue point (3.0 vs. 0.0 points, P=0.004), lower blue point (4.0 vs. 0.0 points, P=0.019), and diaphragmatic point (3.0 vs. 0.0 points, P=0.032) of the non-survivors were significantly higher than those of the survival group. The incidences of the alveolar-interstitial syndrome (100.0% vs. 29.0%, P=0.005) and pleural effusion (80.0% vs. 19.4%, P=0.015) in non-survivors were significantly higher than those of the survivors. The diameter of the IVC value on inhalation in non-survivors was higher than that in surviving patients (10.6 vs. 6.4 mm, P=0.001). However, non-survivors have a lower IVC collapsibility index (0.3 vs. 0.5, P=0.048) than the survivors. No significant differences in LVEF, TAPSE, or MAPSE were found between the two groups (P>0.05).
Table 5
| Parameters | Survivor (n=31) | Non-survivor (n=5) | P value |
|---|---|---|---|
| LUS findings | |||
| LUS score | 7.0 (3.0–10.0) | 17.0 (10.0–24.0) | 0.003 |
| Upper blue point score | 0.0 (0.0–0.0) | 3.0 (1.0–5.0) | 0.004 |
| Lower blue point | 0.0 (0.0–1.0) | 4.0 (2.0–4.0) | 0.019 |
| Diaphragmatic point score | 0.0 (0.0–1.0) | 3.0 (1.0–5.0) | 0.032 |
| Posterior blue points score | 3.0 (1.0–6.0) | 6.0 (2.0–6.0) | 0.396 |
| PLAPS points score | 3.0 (1.0–6.0) | 6.0 (2.0–6.0) | 0.282 |
| Alveolar-interstitial syndrome | 9 (29.0) | 5 (100.0) | 0.005 |
| Pleural effusion | 6 (19.4) | 4 (80.0) | 0.015 |
| Lung consolidations | 26 (83.9) | 5 (100.0) | 1.000 |
| Shred signs | 25 (80.6) | 4 (80.0) | 1.000 |
| Tissue-like sign | 2 (6.5) | 2 (40.0) | 0.084 |
| Where the lung consolidations appeared | 0.011 | ||
| No B lines | 5 (16.1) | 0 (0.0) | |
| Upper blue point | 0 (0.0) | 2 (40.0) | |
| Lower blue point | 4 (12.9) | 1 (20.0) | |
| Diaphragmatic point | 1 (3.2) | 1 (20.0) | |
| Posterior blue points | 13 (41.9) | 1 (20.0) | |
| PLAPS points | 8 (25.8) | 0 (0.0) | |
| Echocardiography findings | |||
| LVEF | 55.0 (50.0–60.0) | 55.0 (48.0–63.0) | 0.379 |
| IVCe (mm) | 15.8±3.5 | 18.1±5.6 | 0.224 |
| IVCi (mm) | 6.4 (5.5–12.6) | 10.6 (9.0–18.7) | 0.001 |
| cIVC | 0.5±0.2 | 0.3±0.2 | 0.048 |
| TAPSE (mm) | 21.1±3.9 | 18.2±6.1 | 0.174 |
| MAPSE (mm) | 13.2±3.7 | 12.0±2.3 | 0.500 |
The continuous values were presented as the means ± standard deviation or median (quartiles) according to their distribution; the categorical variables were presented as counts (percentages). COVID-19, coronavirus disease 2019; LUS, lung ultrasound; PLAPS, posterolateral alveolar and/or pleural syndrome; LVEF, left ventricular ejection fraction; IVCe, inferior vena cava maximal diameter during expiration; IVCi, inferior vena cava maximal diameter during inhalation; cIVC, collapsibility index of inferior vena cava; TAPSE, tricuspid annular plane systolic excursion; MAPSE, mitral annular plane systolic excursion.
Discussion
Although most COVID-19 cases remain moderate symptoms, severe cases can progress to pneumonia, acute respiratory distress syndrome, and death (19). At present, there is no specific treatment for COVID-19, mainly isolation and symptomatic supportive treatment (20). CT is the most effective way to screen and clinically diagnose COVID-19 pneumonia (11). However, the high risk of transporting ventilated patients limits CT availability (21). LUS was effectively used to diagnose acute respiratory distress syndrome, monitor the response to treatment, and detect bacterial superinfection (22,23), as well as COVID-19 pneumonia (24,25). There is a paucity of evidence on assessing the association between LUS characteristics and the severity of COVID-19. Studying the clinical and ultrasound characteristics of COVID-19 help understand the features of critical conditions and promote evaluation and therapeutical decisions.
The LUS score of the lethal cases was higher than that of the survivors in the present study. Our findings are consistent with previous reports on diffuse B lines and subpleural consolidation in critically ill patients (26,27), indicating the more severe pulmonary inflammatory exudation and more abundant mucus in severe/critical cases. On the contrary, the reduced B lines suggested the recovery of gas exchange, underlying the amelioration of consolidations or air-bronchogram reappearance (28,29). Therefore, the features on each part of the lung are conducive for the treatment such as diuresis, mechanical ventilatory support, pleural effusion drainage, and sputum drainage (26).
The incidences of alveolar-interstitial syndrome and lung consolidations in severe/critical patients were higher than those of moderate patients. “Alveolar-interstitial syndrome” showed up in the context of worsened alveolar edema, exudates, and lymphocyte infiltration filling the interstitial space (26,30). Monitoring LUS characteristics could guide continuous positive airway pressure therapy by adjusting the weaning time. In the present study, the proportion of pleural effusion was higher in the lethal cases, indicating that pleural effusions might be related to refractory respiratory failure. It was proven that pleural effusion predicted poor prognosis in H5N1 infection (31), which was similar to the CT distribution of COVID-19 lung lesions reported by Chung et al. (32). It suggested that more attention be paid to combining LUS with clinical values including arterial blood gas to detect the deterioration into severe/critical cases in early stage (11,19,21,22,24,33).
The present study found that the index of the IVC of the severe/critical patients was higher than that of the moderate group because the severe/critical patients had higher blood volume and intrathoracic pressure. It is suggested that attention should be paid to the management of patient’s blood volume, and comprehensive measures should be taken to treat patients to reduce mortality (34). Although the confirmation of COVID-19 pneumonia cannot be achieved via ultrasound, the abovementioned ultrasound characteristics might avail the identification of diverse pathogens and the triage of patients.
Lymphocyte counts in severe/critical cases usually decrease, while WBCs increase. The decrease of lymphocytes indicates the large consumption of immune cells and suppressed immune function. The increased values of the neutrophil ratio and CRP may be associated with the cytokine storm caused by virus invasion and other infectious comorbidities (35). Severe viral infections may cause systemic loss by affecting the balance of pro-inflammatory and anti-inflammatory and inducing the secretion of inflammatory cytokines (36). The worsening lung patterns could be exposed to LUS score, prompting the administration of immunomodulatory therapy. Timely intervention may help reduce complications and mortality. In the present study, LDH, AST, BUN, APTT, and CK-MB generally increased. The changes were more obvious in the severe/critical than in the moderate patients, indicating that severe/critical cases had more severe cardiac, hepatic, renal, and coagulation impairment, which was consistent with previous studies (37,38).
There were still limitations in our study. First, the small size of the study population hindered the exploration of whether the severity of abnormal LUS findings could predict the prognosis for COVID-19 pneumonia. Due to the lack of research, we were unable to analyze the relationship between ultrasound findings and the course of the disease. Second, LUS was performed either in ICU or an isolated ward, resulting in a poor-quality imaging system and incomplete ultrasound data. Third, the demographics were rather simple and lacked relevant characteristics such as cigarette smoking and comorbidities. Also, data on the length of hospital stays and length of ICU stays were missing. Intravenous albumin infusion was given to patients who were prone to turn severe/critical cases, which might result in a higher level in the severe/critical group than in the moderated group in the baseline. More evidence is needed to provide more ultrasound information related to clinical manifestations.
Conclusions
Severe/critical COVID-19 pneumonia demonstrated higher BUN, albumin, CK-MB, NT-proBNP, CRP, and LDH level, as well as higher SOFA scores than moderate cases. The study demonstrated that IVC and LUS score in admission was significantly different in severe patients compared with moderate patients. Patients in severe/critical cases had more alveolar-interstitial syndrome and lung consolidations. The abovementioned features help quantify the severity of COVID-19.
Acknowledgments
Funding: This study was supported by the 2020 National Natural Science Foundation of China Start-Up Funding (Youth Project) (No. KY012020267).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aoi.amegroups.com/article/view/10.21037/aoi-22-4/rc
Data Sharing Statement: Available at https://aoi.amegroups.com/article/view/10.21037/aoi-22-4/dss
Peer Review File: Available at https://aoi.amegroups.com/article/view/10.21037/aoi-22-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aoi.amegroups.com/article/view/10.21037/aoi-22-4/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First People’s Hospital of Jingzhou and Guangdong Provincial People’s Hospital (No. 2020-056), and written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:4. [Crossref] [PubMed]
- Kim YC, Jeong BH. Strong Correlation between the Case Fatality Rate of COVID-19 and the rs6598045 Single Nucleotide Polymorphism (SNP) of the Interferon-Induced Transmembrane Protein 3 (IFITM3) Gene at the Population-Level. Genes (Basel) 2020;12:42. [Crossref] [PubMed]
- Liu KC, Xu P, Lv WF, et al. Differential diagnosis of coronavirus disease 2019 from community-acquired-pneumonia by computed tomography scan and follow-up. Infect Dis Poverty 2020;9:118. [Crossref] [PubMed]
- Xu G, Yang Y, Du Y, et al. Clinical Pathway for Early Diagnosis of COVID-19: Updates from Experience to Evidence-Based Practice. Clin Rev Allergy Immunol 2020;59:89-100. [Crossref] [PubMed]
- Pham TD. A comprehensive study on classification of COVID-19 on computed tomography with pretrained convolutional neural networks. Sci Rep 2020;10:16942. [Crossref] [PubMed]
- Fields BKK, Demirjian NL, Dadgar H, et al. Imaging of COVID-19: CT, MRI, and PET. Semin Nucl Med 2021;51:312-20. [Crossref] [PubMed]
- Kulkarni S, Down B, Jha S. Point-of-care lung ultrasound in intensive care during the COVID-19 pandemic. Clin Radiol 2020;75:710.e1-4. [Crossref] [PubMed]
- Mojoli F, Bouhemad B, Mongodi S, et al. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med 2019;199:701-14. [Crossref] [PubMed]
- Brusasco C, Santori G, Bruzzo E, et al. Quantitative lung ultrasonography: a putative new algorithm for automatic detection and quantification of B-lines. Crit Care 2019;23:288. [Crossref] [PubMed]
- Smith MJ, Hayward SA, Innes SM, et al. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia 2020;75:1096-104. [Crossref] [PubMed]
- Zu ZY, Jiang MD, Xu PP, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020;296:E15-25. [Crossref] [PubMed]
- Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J Ultrasound Med 2020;39:1413-9. [Crossref] [PubMed]
- Miller DL, Dong Z, Dou C, et al. Pulmonary Capillary Hemorrhage Induced by Different Imaging Modes of Diagnostic Ultrasound. Ultrasound Med Biol 2018;44:1012-21. [Crossref] [PubMed]
- Moore S, Gardiner E. Point of care and intensive care lung ultrasound: A reference guide for practitioners during COVID-19. Radiography (Lond) 2020;26:e297-302. [Crossref] [PubMed]
- Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374. [Crossref] [PubMed]
- Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147:1659-70. [Crossref] [PubMed]
- Demi M, Prediletto R, Soldati G, et al. Physical Mechanisms Providing Clinical Information From Ultrasound Lung Images: Hypotheses and Early Confirmations. IEEE Trans Ultrason Ferroelectr Freq Control 2020;67:612-23. [Crossref] [PubMed]
- Liu X, Liu C, Liu G, et al. COVID-19: Progress in diagnostics, therapy and vaccination. Theranostics 2020;10:7821-35. [Crossref] [PubMed]
- Torres Acosta MA, Singer BD. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur Respir J 2020;56:2002049. [Crossref] [PubMed]
- Bai H, Ji Y, Wang J, et al. Efficacy of human coronavirus immune convalescent plasma for the treatment of corona virus disease -19 disease in hospitalized children: A protocol for systematic review and meta analysis. Medicine (Baltimore) 2020;99:e22017. [Crossref] [PubMed]
- Farias LPG, Fonseca EKUN, Strabelli DG, et al. Imaging findings in COVID-19 pneumonia. Clinics (Sao Paulo) 2020;75:e2027. [Crossref] [PubMed]
- Zhang YK, Li J, Yang JP, et al. Lung ultrasonography for the diagnosis of 11 patients with acute respiratory distress syndrome due to bird flu H7N9 infection. Virol J 2015;12:176. [Crossref] [PubMed]
- Testa A, Soldati G, Copetti R, et al. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care 2012;16:R30. [Crossref] [PubMed]
- Kanne JP, Little BP, Chung JH, et al. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology 2020;296:E113-4. [Crossref] [PubMed]
- Pronyuk KO, Kondratiuk LO, Vysotskyi AD, et al. Lung ultrasound during covid-19 pandemics: why, how and when? Wiad Lek 2021;74:1783-8. [Crossref] [PubMed]
- Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020;295:715-21. [Crossref] [PubMed]
- Kameda T, Mizuma Y, Taniguchi H, et al. Point-of-care lung ultrasound for the assessment of pneumonia: a narrative review in the COVID-19 era. J Med Ultrason (2001) 2021;48:31-43. [PubMed]
- Pichamuthu K. Lung Ultrasound: COVID-19's Silver Lining. Indian J Crit Care Med 2021;25:8-9. [Crossref] [PubMed]
- Lesser FD, Smallwood N, Dachsel M. Point-of-care lung ultrasound during and after the COVID-19 pandemic. Ultrasound 2021;29:140. [Crossref] [PubMed]
- Soldati G, Smargiassi A, Inchingolo R, et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J Ultrasound Med 2020;39:1459-62. [Crossref] [PubMed]
- Qureshi NR, Hien TT, Farrar J, et al. The radiologic manifestations of H5N1 avian influenza. J Thorac Imaging 2006;21:259-64. [Crossref] [PubMed]
- Chung M, Bernheim A, Mei X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020;295:202-7. [Crossref] [PubMed]
- Bitar ZI, Shamsah M, Maadarani O, et al. Lung Ultrasound and Sonographic Subpleural Consolidation in COVID-19 Pneumonia Correlate with Disease Severity. Crit Care Res Pract 2021;2021:6695033. [Crossref] [PubMed]
- Bitar ZI, Shamsah M, Bamasood OM, et al. Point-of-Care Ultrasound for COVID-19 Pneumonia Patients in the ICU. J Cardiovasc Imaging 2021;29:60-8. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther 2020;5:128. [Crossref] [PubMed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
Cite this article as: Huang D, Jiang C, Song X, Yuan H, Li F, Wang S, Qin T, Zhang Q, Ma H, Tan X, Song F. Ultrasound and clinical characteristics of patients with COVID-19 pneumonia: a cross-sectional study. Ann Infect 2023;7:1.


