
Management and outcome predictors during Herpes simplex virus encephalitis
Introduction
Worldwide, Herpes simplex virus encephalitis (HSE) is the most common cause of sporadic acute viral encephalitis in adults (1). It has an incidence between 1 and 3 cases per million individuals, a mortality rate up to 30% and a high incidence of severe and permanent neurologic sequelae (2).Most cases (90%) are attributed to infection by Herpes simplex virus (HSV) type 1, while 7% are caused by HSV type 2, which have traditionally shown a more indolent form of central nervous system (CNS) involvement (3,4). It is thought that HSE results from a recent infection or reactivation of latent HSV genomes residing in certain CNS sites (2,3).
The diagnostic testing had evolved during these few last years. The key to establishing evidence of CNS inflammation is the analysis of cerebrospinal fluid (CSF). Lumbar puncture is often delayed due to performing brain imaging to exclude raised intracranial pressure (5). The application of polymerase chain reaction (PCR) for the detection of HSV genetic material (DNA) in the CSF provided the gold standard for the diagnosis of HSE (6).Magnetic resonance imaging (MRI) is the imaging of choice when encephalitis is suspected (5). Even with antiviral therapy, survival rates remain at 70% and life-long neurological deficits and sequelae such as anterograde amnesia, difficulties with executive function and aphasia are often reported (7).
The prognosis of HSE remains poor, especially if not promptly diagnosed. However, other factors may interfere and lead to a severe form of HSE. A better knowledge of this disease and its outcome predictors may help clinicians through the management of HSE. In this perspective, the aim of this work was to identify the epidemiological, clinical, evolutionary features and to study the outcome predictors of HSE.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/aoi-20-14).
Methods
Study design
We carried out a retrospective study including all patients hospitalized for HSE in the infectious diseases department over a 25-year period between January 1994 and December 2018.
Data collection and case definition
Patients included in our study had a possible, probable or confirmed encephalitis based on the diagnostic criteria of the Consensus Statement of the International Encephalitis Consortium (8). Those criteria were based on a major criterion (required component) represented by an alteration of the mental status. It was defined as decreased or altered level of consciousness, lethargy or personality change lasting ≥24 hours with no alternative cause identified. Minor criteria included:
- Onset of fever more than 38 °C within the 72 hours before or after presentation;
- Generalized or partial seizures which are not totally related to a pre-existing seizure disorder;
- New onset of focal neurologic findings;
- White blood cells (WBCs) count more than 5/mm3 in the CSF;
- Neuroimaging showing abnormality of brain parenchyma which suggests encephalitis that is either new from prior studies or appears acute in onset;
- Abnormal electroencephalography that suggests encephalitis and not related to another etiology.
A major criterion associated to 2 minor criteria defined a possible encephalitis. Probable or confirmed encephalitis was defined by a major criterion and ≥3 minor criteria. The positivity of HSV-1/2 PCR in the CSF defined patients with confirmed encephalitis.
Data were collected from patients’ medical record on pre-established sheets. We recorded demographic data, duration and characteristics of clinical symptoms and physical examination findings. We recorded analysis of the CSF results including PCR detection of HSV DNA, electroencephalography, brain computed tomography (CT) and MRI. The delay between the onset of clinical signs and initiation of treatment, its regimen and duration, side effects and outcome were noted. We scored the level of morbidity as:
- No sequelae: the patient’s health is exactly like the pre-encephalitic period;
- Mild sequelae: minor disability compared to pre-encephalitic stage such as memory impairment or decrease in attention span. Ability to work and function autonomously;
- Moderate sequelae: subjective major disability compared to pre-encephalitic stage, mostly self-sufficient in daily routine. Significant differences compared to the pre-encephalitic stage were noted. The patient can’t maintain his job;
- Severe sequelae: when there is a major neuropsychiatric disability or chronic care. The patient needs constant help for daily routine.
The prognosis was defined favourable in the absence of sequelae or in case of mild or moderate sequelae. Poor prognosis was associated to severe sequelae or death.
Due to retrospectively obtained data of the study, ethical approval and the informed patient consent were not required.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Statistical analysis was performed using SPSS 20. Qualitative variables were carried out by numbers and percentages. When the distribution was normal, quantitative variables were presented by means and standard deviation. Otherwise, medians and interquartile ranges were performed. The t test was used to compare two means if they were normally distributed. Chi square test was used for categorical variables in independent samples. Wilcoxon, Mann-Whitney, and Kruskal-Wallis tests were used when the variables were not normally distributed. The receiver operating characteristic (ROC) methodology was performed to evaluate the hospitalization delay or the treatment delay predicting poor prognosis. The optimal cutoff value defined as the value with the highest sensitivity and specificity was determined. The area under the ROC curve, sensitivity and specificity were calculated. The difference between two groups was considered to be significant when P<0.05.
Results
Patients’ characteristics
During the study period, 225 patients were hospitalized for meningoencephalitis or encephalitis, among whom 30 patients had HSE, representing therefore 13.3% of all cases. Fifteen patients were male (50%). The mean age was 44±16 years. Nine patients were initially admitted to the intensive care unit and transferred later to the infectious disease department (30%). The median duration between the onset of symptoms and hospitalisation was 4 days (1–21 days). The most common clinical features were fever (96.6%), cephalalgia (70%) and aggressive behavior (63.3%). Physical examination revealed, besides fever (96.6%), meningeal syndrome in 19 cases (63.3%), confusion in 11 cases (36.7%) and obnubilation in 10 cases (33.3%) (Table 1).
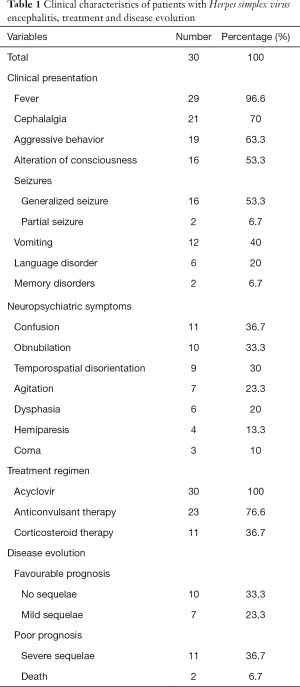
Full table
Lumbar puncture was performed in all cases (100%). Analysis of CSF revealed an elevated WBC count (>5/mm3) in 26 cases (86.6%) with a median of 54/mm3 (5–500/mm3). There was lymphocyte-predominant pleocytosis in 25 cases (96.1%), while neutrophil-predominant pleocytosis was noted in one case (3.9%). The median level of protein was 0.51 g/L (0.1–4.1 g/L). Seventeen patients (56.6%) had an increased level of protein (>0.45 g/L). The CSF glucose level was within the reference range in 27 cases (90%) and low in three cases (10%). Control lumbar puncture was indicated in 23 cases (76.6%) after a median duration of 9 days (2–24 days). In total, HSV PCR assay was performed in the CSF in 15 cases (50%) after a median duration of 5 days (2–12 days). Eleven patients had a positive HSV PCR (73.3%). The four remaining patients with a negative HSV PCR were already treated with acyclovir when lumbar puncture was performed (26.7%).
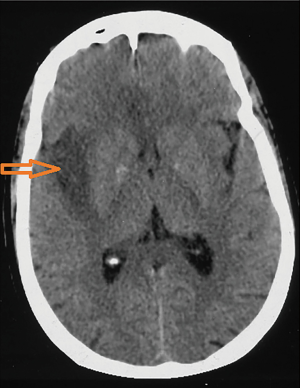
An electroencephalogram (EEG) was performed in 20 cases (66.6%) after a median duration of 9 days (2–150 days). Diffuse slowing was the most common abnormality noted in 65% of the cases. Periodic and non-periodic sharp waves were noted in three cases (15%) and two cases (10%), respectively. It was normal in two cases (10%). Brain CT scan, performed in 21 cases (70%), demonstrated parenchymal hypodensity in 14 cases (66.6%) (Figure 1). Hypodensity was located in the temporal lobe in seven cases (50%). It revealed mass effect in three cases (14.3%) and diffuse cerebral edema in one case (4.8%).
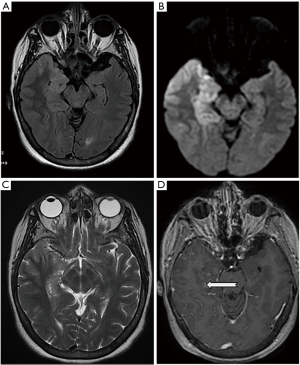
Brain MRI was performed in 27 cases (90%), among which 24 cases were pathological (88.8%). Temporal involvement was characteristic in 18 cases (66.6%). The observed lesions were unilateral in 15 cases (55.6%) and bilateral in nine cases (33.3%). Three cases had a normal MRI (11.1%). Lesions with low signal on T1 were noted in 18 cases (66.6%). Nineteen patients had lesions with high signal on T2 (70.3%) (Figure 2).
All patients received intravenous acyclovir for a mean duration of 19±7 days. The mean delay from the onset of encephalitis signs to initiation of treatment was 5±2 days. Empiric therapy was initiated on admission prior to confirmation of the diagnosis in 18 patients (60%). Side effects were noted in 5 cases (16.6%). Corticosteroid therapy was prescribed in 8 cases (26.7%) for a mean duration of 9±3 days. In order to treat or to prevent seizures, anticonvulsant therapy was prescribed in 23 cases (76.6%). It was continued after discharge in 10 cases (33.3%). The disease evolution was marked by the occurrence of recovery without sequelae in 10 cases (33.3%). Sequelae were noted in 18 cases (60%) represented by mild sequelae in 7 cases (23.3%) and severe sequelae in 11 cases (36.7%). Two patients were dead (6.7%). In total, there were 17 cases with a favourable prognosis (56.6%) and 13 cases with a poor prognosis (43.3%).
Outcome and prognostic factors for Herpes simplex virus encephalitis
Comparison of the disease evolution showed that poor prognosis was significantly more frequent in patients hospitalized after a delay of 3 days after the onset of symptoms [odds ratio (OR) =13.5 (1.4–80.2); P=0.017]. Studying the physical examination signs on admission and the prognosis, we found that only hemiparesis was significantly more frequent in patients with a poor prognosis (P=0.02). The poor prognosis was significantly more frequent when hypoglycorrhachia less than 3.3 mmol/L was noted in CSF [OR =10.5 (1.8–58.3); P=0.008]. Starting acyclovir therapy after a delay of 3 days was significantly more associated with a poor prognosis [OR =10.6 (1.2–74.3); P=0.04] (Table 2).
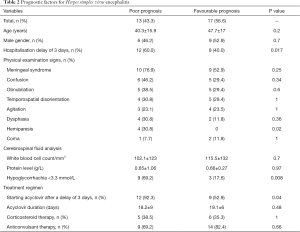
Full table
The analysis of the ROC curve showed that for a threshold value of 3 days between the onset of clinical signs and hospitalization, the sensitivity was 92% and the specificity was 53% to predict a poor prognosis in patients having HSE (Figure 3).
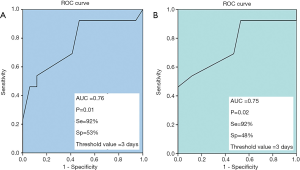
The analysis of the ROC curve showed that for a delay of 3 days between starting antiviral treatment and the onset of encephalitis signs, the sensitivity and the specificity to predict a poor prognosis in patients with HSE were 92% and 48%, respectively (Figure 3).
Discussion
Our study highlighted the burden of HSE, which remains a fatal, life threatening disease associated with a poor prognosis and neurological sequelae especially when the diagnosis and the treatment were delayed. The factors associated with a poor prognosis were hospitalization delay of 3 days after the onset of symptoms, hemiparesis noted on physical examination, hypoglycorrhachia in CSF and starting antiviral treatment with a delay of 3 days. It was not a rare disease, representing 13.3% of all meningoencephalitis cases in our study.
The revealing symptoms varied widely. They are not pathognomonic of HSE and might reveal other causes of acute encephalitis (9). Many patients present with viral-like prodromal symptoms including headache, fever (10), behavioral changes and occasionally olfactory hallucinations (11). Then, signs of encephalitis progress over the course of several days (12). The most common manifestations were altered mentation, reduced level of consciousness, fever, seizures, headaches and focal neurological deficits including cranial nerve deficits, hemiparesis, dysphasia, aphasia or ataxia (11,12). Other studies found that in younger children, the initial symptoms of the disease are usually insomnia, irritability, movement disorders or epileptic seizures, while psychiatric disorders are less common (10). Depending on their clinical presentation, patients may require admission to the intensive care unit. A study made in Texas found that most hospitalizations for HSE were admitted to intensive care unit (59.9%) among which 45.8% were aged ≥65 years (13). In our study, 30% of patients were initially admitted to the intensive care unit. Despite the well-described clinical features of HSE in different studies, they remain non-specific and might mimic other diseases.
Once the diagnosis is suspected and after ruling out contraindications, lumbar puncture should be performed as soon as possible. Typical CSF abnormalities include elevated protein, CSF leukocytosis with predominance of lymphocytes (60% to 98%). The absence of CSF leukocytes should not eliminate the diagnosis since cases of HSE without CSF leukocytosis have been reported (11).Hemorrhagic CSF is not specific to HSE since hemorrhagic encephalitis can also be seen in varicella zoster, rubella, Epstein-Barr virus or amoeba infection in immunocompromised patients (14). As for CSF glucose value, it is usually normal, although cases of hypoglycorrhachia have been previously reported (11), which is consistent with our results.
The cornerstone of the diagnosis is PCR detection of HSV DNA in CSF. However, at an early stage of the disease, PCR can be false negative (15), that’s why it has to be repeated within 48 to 72 hours in patients with suspected encephalitis (16). In our study, 26.7% of patients had a negative HSV PCR, but they were already treated with acyclovir when lumbar puncture was performed, that’s why no control was made. Previous studies reported that cases with initially negative, but subsequently positive PCR on a repeat CSF testing had positive MRI signs compatible with HSE at the time of initial negative PCR (15).
Neuroimaging is always indicated. As for CT scanning, it is usually normal within the first 4 to 6 days of disease. However, it is basically indicated in the presence of focal neurologic signs, before lumbar puncture in order to rule out contraindications (2). MRI can aid in the diagnosis of HSE by demonstrating typical imaging findings, which include asymmetric hyperintense lesions on T2-weighted sequences (12). MRI is considered the most sensitive and specific imaging method for the diagnosis, particularly early in the course of the illness (12). However, up to 5% of patients with HSE have a normal MRI (17). Therefore, imaging alone should not exclude the diagnosis of encephalitis. During the diagnostic process, EEG may play a major role especially in front of atypical clinical findings and potential negative laboratory examinations. In the acute stage, EEG can show lateralized periodic discharges, sharp waves and focal or generalized slowing. These patterns can reflect the focality, extent of the disease and may even inform about the prognosis (18). In our study, diffuse slowing was the most common abnormality (65%).
When clinical, laboratory, radiographic and neurophysiologic findings suggest the diagnosis of HSE, empirical treatment should be initiated with no delay. Acyclovir should be administered at a dose of 10 mg/kg/8 hours for 14 days in immunocompetent patients (19). Early initiation of acyclovir is the most readily modifiable factor for improving outcomes (12). Previous studies reported that an unfavourable outcome both at discharge and at one year was associated with a delay in the initiation of acyclovir after admission and longer delays had the most serious neurological consequences (4). Other studies reported that older age, coma, restricted diffusion on MRI and delay in the initiation of acyclovir were the factors associated with the worse outcome (9).
The use of corticosteroids in the treatment of HSE is controversial since it may potentiate viral replication and cell damage if they are given at a too early stage. However, most authors seem to support the delayed use of corticosteroids following the introduction of an antiviral therapy in intracranial hypertension due to HSE. Its efficacy was demonstrated in several retrospective studies (20). Besides, anticonvulsant therapy is often indicated in order to treat seizures, since HSE is highly epileptogenic (21).
Conclusions
Our study confirmed the severity of HSE cases and its poor prognosis mainly associated to diagnosis and treatment delay. Studying the prognostic factors may help clinicians in the management of cases of HSE. In front of febrile altered level of consciousness, the diagnosis of HSE should be strongly considered and prompt empiric acyclovir treatment should be started in order to improve the prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/aoi-20-14
Data Sharing Statement: Available at https://dx.doi.org/10.21037/aoi-20-14
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aoi-20-14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Due to retrospectively obtained data of the study, ethical approval and the informed patient consent were not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duarte LF, Farías MA, Álvarez DM, et al. Herpes Simplex Virus Type 1 Infection of the Central Nervous System: Insights Into Proposed Interrelationships With Neurodegenerative Disorders. Front Cell Neurosci 2019;13:46. [Crossref] [PubMed]
- Steiner I, Benninger F. Manifestations of Herpes Virus Infections in the Nervous System. Neurol Clin 2018;36:725-38. [Crossref] [PubMed]
- Berger A, Shahar T, Margalit N. Herpes Simplex Type 2 Encephalitis After Craniotomy: Case Report and Literature Review. World Neurosurg 2016;88:691.e9-691.e12. [Crossref] [PubMed]
- Singh TD, Fugate JE, Hocker S, et al. Predictors of outcome in HSV encephalitis. J Neurol 2016;263:277-89. [Crossref] [PubMed]
- Ellul M, Solomon T. Acute encephalitis - diagnosis and management. Clin Med (Lond) 2018;18:155-9. [Crossref] [PubMed]
Whitley R Baines J Clinical management of herpes simplex virus infections: past, present, and future. F1000Res 2018 ;7. doi: .10.12688/f1000research.16157.1 - Menendez CM, Carr DJJ. Defining nervous system susceptibility during acute and latent herpes simplex virus-1 infection. J Neuroimmunol 2017;308:43-9. [Crossref] [PubMed]
- Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis 2013;57:1114-28. [Crossref] [PubMed]
- Rabinstein AA. Herpes Virus Encephalitis in Adults: Current Knowledge and Old Myths. Neurol Clin 2017;35:695-705. [Crossref] [PubMed]
- Guasp M, Dalmau J. Encephalitis associated with antibodies against the NMDA receptor. Med Clin (Barc) 2018;151:71-9. [Crossref] [PubMed]
- Gnann JW Jr, Whitley RJ. Herpes Simplex Encephalitis: an Update. Curr Infect Dis Rep 2017;19:13. [Crossref] [PubMed]
- Bradshaw MJ, Venkatesan A. Herpes Simplex Virus-1 Encephalitis in Adults: Pathophysiology, Diagnosis, and Management. Neurotherapeutics 2016;13:493-508. [Crossref] [PubMed]
- Oud L. Herpes Simplex Virus Encephalitis: Patterns of Epidemiology and Outcomes of Patients Admitted to the Intensive Care Unit in Texas, 2008 - 2016. J Clin Med Res 2019;11:773-9. [Crossref] [PubMed]
- Parlak E, Erturk A, Karaca L. Early diagnosis of herpes encephalitis saves lives: A case report. J Exp Clin Med 2015;32:35-8.
- Sili U, Kaya A, Mert A, et al. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol 2014;60:112-8. [Crossref] [PubMed]
- Ghannad MS, Solgi G, Hashemi SH, et al. Herpes simplex virus encephalitis in hamadan, iran. Iran J Microbiol 2013;5:272-7. [PubMed]
- Baldwin KJ, Cummings CL. Herpesvirus Infections of the Nervous System. Continuum (Minneap Minn) 2018;24:1349-69. [Crossref] [PubMed]
- Baten A, Desai M, Melo-Bicchi M, et al. Continuous Electroencephalogram as a Biomarker of Disease Progression and Severity in Herpes Simplex Virus-1 Encephalitis. Clin EEG Neurosci 2019;50:361-5. [Crossref] [PubMed]
- Stahl JP, Mailles A. Herpes simplex virus encephalitis update. Curr Opin Infect Dis 2019;32:239-43. [Crossref] [PubMed]
- Todeschi J, Gubian A, Wirth T, et al. Multimodal management of severe herpes simplex virus encephalitis: A case report and literature review. Neurochirurgie 2018;64:183-9. [Crossref] [PubMed]
- Halperin JJ. Diagnosis and management of acute encephalitis. Handb Clin Neurol 2017;140:337-47. [Crossref] [PubMed]
Cite this article as: Hammami F, Koubaa M, Ellouze Y, Feki W, Rekik K, Smaoui F, Elleuch E, Marrakchi C, Ben Jemaa M. Management and outcome predictors during Herpes simplex virus encephalitis. Ann Infect 2021;5:5.


