
Atypical transmission of Epstein-Barr virus to a medical practitioner—a case report
Introduction
Infectious mononucleosis (IM) is classically characterised by a triad of tonsillar pharyngitis, fever and lymphadenopathy. About 90% of IM cases are attributed to Epstein-Barr virus (EBV) with the remainder precipitating from other viral pathogens such as cytomegalovirus (CMV) and human herpesvirus 6 (1). In many cases, EBV infects children who demonstrate very few or no symptoms. In young adults, the classic symptoms encompass fever, sore throat, fatigue and lymphadenopathy. Most individuals recover within 4 weeks, but fatigue may persist for months. Uncommon findings include morbilliform or maculopapular rashes, palatal petechiae, rhinitis and as observed in this case, periorbital oedema which is known as the Hoagland’s sign (2).
Oral transmission through the exchange of saliva is the major route of transmission for primary EBV but infection from exposure to aerosolised droplets is not widely documented in the literature (3).
Given the highly variable clinical manifestations in the early stages of EBV infections, medical practitioners are often left in a diagnostic quandary. Here, we seek to expand the current knowledge base by illustrating a unique case of EBV infection manifesting atypically with Hoagland’s Sign and rhinitis, and where transmission to an immunocompetent healthcare worker has occurred through exposure to respiratory droplets (via sneezing). We hope this case will elicit discussion about the mandatory use of masks when interacting with patients suspected or confirmed to have an acute EBV infection.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aoi-21-5).
Case presentation
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Mr A is a 26-year-old immunocompetent medical practitioner who presented to the general practitioner (GP) with a 5-day history of bilateral upper-palpebral oedema (Figure 1), epiphora and coryzal symptoms. He was otherwise completely well with no significant past medical or family history. Routine laboratory findings were all within the normal limits, apart from a radioallergosorbent test which demonstrated a very high IgE response 42.70 KU/L (<0.35 KU/L) to house dust and mites, specifically Dermatophagoides pteronyssinus. He was subsequently diagnosed with allergic rhinitis and was recommended to use fexofenadine 180 mg daily and antihistamine eye drops twice daily.
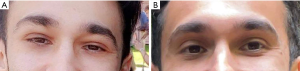
Over the next 4 days, the bilateral upper-palpebral oedema worsened (Figure 1A) and was then accompanied by severe headaches and early morning vomiting. Mr. A then presented to an emergency department where a routine computerized tomography scan of the brain and sinuses was performed, demonstrating no abnormalities accounting for his symptoms. He was subsequently discharged back to the GP for ongoing care.
Mr. A re-presented to his GP, 12 days following the onset of the initial symptoms, with fevers, arthralgias, pallor, anorexia, loss of weight and fatigue. Given the progression of his symptoms, the GP revisited the history and on targeted questioning, Mr. A revealed that whilst working in the emergency department of a major tertiary hospital, a patient (who was subsequently tested newly infected with EBV) had sternutated in the consultation room, 30 days prior to the onset of his initial symptoms. Given this case occurred pre-COVID-19, Mr. A had not worn any personal protective equipment (PPE) prior to patient contact. No other possible means of EBV transmission were identified. On examination, posterior cervical and occipital lymphadenopathy was noted, and abdominal palpation demonstrated marked splenomegaly. Repeat laboratory findings demonstrated thrombocytopenia (137×109/L), neutropenia (1.4×109/L), and an elevation in C-reactive protein (14 mg/L), ferritin (442 µmol/L) and alanine aminotransferase (68 U/L). A lymphocyte to white cell count ratio was 56.9%, suggestive of IM. Unfortunately, a peripheral blood smear was not obtained to determine atypical lymphocytes. A monospot test was positive for IgM antibodies and EBV viral capsid antigens (VCA) IgM and EBV VCA IgG antibodies were also positive. EBV nuclear antigen (NA) IgG antibodies and CMV serology were negative, demonstrating an active EBV infection in a previously unexposed patient. A gastroenterologist was consulted regarding the laboratory findings and a definitive diagnosis of EBV infection was established. Repeat EBV serology in 1–2 months was advised to ensure EBV NA seroconversion. An abdominal ultrasound confirmed moderate splenomegaly, whereby the spleen measured 16.5 cm × 11.3 cm × 12.2 cm with an approximate volume of 1,182 cc (Figure 2A), with the absence of hepatomegaly. He was recommended to avoid contact sports for 1 month and to take ibuprofen for arthralgias when required. The following week, Mr. A re-presented to the GP with severe throat pain and odynophagia. His previous symptoms had completely resolved, and physical examination revealed an exudative grade III tonsillitis. The patient was advised to gargle saltwater and continue with ibuprofen for pain. The tonsilitis resolved after 5 days and the patient was booked in for follow-up investigations in a month.
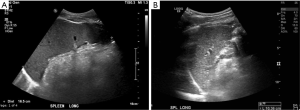
Our patient returned to the GP clinic after 1 month to undergo a repeat abdominal ultrasound and serological testing. The patient was asymptomatic (Figure 1B), and all routine bloods had normalised. EBV VCA IgM and EBV VCA IgG antibodies were still positive, whilst EBV NA IgG antibodies were negative, indicating seroconversion had not yet occurred. A repeat abdominal ultrasound demonstrated resolution of splenomegaly, with the spleen now measuring 10.4 cm (Figure 2B).
After 11 months, the patient returned for routine blood tests and EBV serology indicated seroconversion and demonstrated evidence of past infection with positive EBV NA IgG antibodies and negative EBV VCA IgM antibodies.
Discussion
EBV, also known as human herpesvirus 4, is a lymphotropic herpesvirus that is primarily responsible for the syndrome of IM. It usually spreads through the exchange of saliva, infecting the oropharyngeal epithelial cells and the naïve B cells of the oral cavity mucosal lymphoid tissues (4). Infected naive B cells undergo differentiation to form circulating pools of latently infected memory B cells which are prone to periodic reactivation, resulting in further viral shedding and recurring infections. Latently infected memory B cells escape detection by CD8+ cytotoxic T cells due to low expression of viral proteins. Even after recovery from IM, viral shedding may continue in salivary secretions for many months.
Primary EBV infection may also be transmitted via blood transfusion, sexual contact, organ transplantation and hematopoietic cell transplantation (5). The Centers for Disease Control and Prevention (6) only stipulates the use of standard precautions when handling patients with EBV infections, due to the aforementioned modes of transmission. Given this case took place prior to COVID-19, there was no mandatory need for PPE with droplet precautions when assessing patients with respiratory symptoms, which may have otherwise prevented transmission in our patient. Until now, it is understandable the hesitancy in using PPE when treating patients suspected or infected with EBV. The cost and time used to administer PPE are factors in which many institutions may wish to avoid use of masks, especially in conditions where there have been no documented instances of transmission via respiratory droplets. An increased use of masks may also impede on communication with patients and families, however, during the COVID-19 era, medical practitioners have adopted techniques and other platforms to overcome this barrier whilst wearing masks with other forms of PPE. Therefore, we strongly advocate the use of masks and practice appropriate hand hygiene in all patients suspected or confirmed to have an acute EBV infection, given the mode of transmission described in our case report.
Typical features of an EBV infection include fatigue, fever, pharyngitis and adenopathy. Two review studies (7) involving a collective of 500 patients, reported lymphadenopathy in all patients, 98% had fever and 85% presented with pharyngitis. These clinical manifestations tend to mirror other viral infections and establishing an accurate initial diagnosis may prove challenging. The Hoagland’s criteria are a widely accepted assessment algorithm used for diagnosing IM (7). It states that in individuals with fever, pharyngitis and adenopathy and distinct blood film features (at least 50% lymphocytes and at least 10% atypical lymphocytes), the diagnosis of IM should be confirmed with serologic testing (7). This diagnostic approach has a specificity of 95% and a sensitivity of 61% (8). Using a lower rate of lymphocytosis yields higher false negatives especially if atypical lymphocytes are disregarded (1). Mr. A fulfilled a majority of Hoagland’s criteria with his lymphocytes above 50% but atypical lymphocyte counts were unavailable.
Mr. A also exhibited atypical initial disease manifestations, namely the bilateral upper-palpebral swelling and rhinitis. The association between transient bilateral upper-palpebral swelling/peri-orbital oedema and IM was first described by Hoagland in 1952 who noted its presence in a third of IM cases (9). This association is referred to as the “Hoagland’s sign”. It occurs very early in the disease process (within days) and often precedes exudative pharyngitis, cervical lymphadenopathy (9) and atypical lymphocytes on differential counts (2). The Hoagland’s sign was an early manifestation in Mr. A and was not associated with blepharitis or conjunctivitis (2). The pathophysiology of Hoagland’s sign remains unestablished but nasopharyngeal viral colonisation, lymphoproliferation and lymphatic blockages have all been implicated (10). Several studies have noted the presence of peri-orbital or bilateral upper-palpebral oedema (though not explicitly defined as the “Hoagland’s sign”) in about 25–35% of IM cases (11). Nevertheless, further research is needed to determine its specificity and sensitivity.
The screening for heterophile antibodies may involve latex agglutination assay which utilises horse erythrocytes as the medium (12). Other quick diagnostic screens use enzyme-linked immunosorbent assay techniques. The specificity for both rapid kits approach 100% but their sensitivity is only up to 85% (13). As a result, clinicians should be cautious in the first week of infection since false negatives can be up to 25% (14). Mr. A had the latex agglutination type screen and an EBV-specific antibody testing on the same day with the latter repeated at scheduled intervals. The EBV-specific antibody should be measured if suspicion for IM is high with a negative monospot test (15). These specific antibody tests detect VCA-IgG, VCA-IgM and EBV NA IgG (14) and have a sensitivity of 97% (16). On the other hand, its specificity is 94% (16) therefore they may be slightly inferior to the heterophile antibody tests in ruling in infection. VCA-IgM and VCA-IgG are made earlier than the heterophile antibody with the latter persisting in the chronic phase (14). EBV NA IgG is usually not detectable until 6 to 12 weeks after the onset of symptoms and is a late marker of EBV infection, thus reflecting disease recovery or previous exposure (4). Nevertheless, VCA-IgG is still the better indicator for previous infection since EBV NA IgG is absent in 5–10% of infections for immunocompetent individuals and even higher numbers of immunocompromised patients fail to have detectable levels (3).
There is no established consensus on how to evaluate patients with suspected IM but one report proposed recommendations collated from the available evidence (14). Ebell concluded that patients between the ages of 10 and 30 years with fever, sore throat, anterior and posterior cervical adenopathy, fatigue, inguinal adenopathy, palatal petechiae, or splenomegaly are at high risk for IM. A white blood cell count with differential or a heterophile antibody test should be done with an additional rapid test for streptococcal pharyngitis. IM is strongly supported if blood film shows more than 20% atypical lymphocytes or more than 50% lymphocytes with at least 10% atypical lymphocytes (14). When symptomatic treatment fails within a week, a second heterophile antibody test should be obtained. If an accurate diagnosis is needed urgently such as for Mr. A who works in healthcare, an EBV-specific measure is an option.
van Hasselt et al. described a case of a 15-year-old girl who presented with a 3-day history of progressive bilateral eyelid swelling despite treatment with an antihistamine. EBV was finally diagnosed after a positive monospot test and the presence of EBV VCA IgM antibodies (17). In another case study, clinicians were able to diagnose EBV in an eight-and-a-half-year-old after recognising the Hoagland’s sign and other coryzal symptoms (2). The diagnosis was later confirmed with serological testing for VCA IgM antibodies allowing for early appropriate supportive management (17). Rhinitis, another uncommon finding in IM has been documented in 10–25% of EBV related infections (11). Clinicians should consider the atypical features such as rhinitis and Hoagland’s sign in the early stages of IM and practise droplet precaution to prevent transmission. Although most patients in literature who had transient bilateral upper-palpebral swelling/peri-orbital oedema were young or in their adolescence, our case demonstrates that it is not only limited to these age groups.
EBV infects at least 90% of the world population and yet there is no approved vaccine available, despite being in the works for years (18). In addition to financial implications of vaccination, the vast majority of infections lead to no complications or long-term morbidity, despite EBV being associated with multiple malignancies. Mainstay of treatment is supportive care with very limited evidence supporting the use of antivirals, corticosteroids or anaerobic antibacterial agents. A meta-analysis of five randomised controlled trials of acyclovir in treating acute IM had shown to be no better than placebo (19). Despite the widespread use of corticosteroids seen in IM cases, a Cochrane Review found a lack of quality evidence supporting its use for symptom control except in cases of airway emergencies (20). As with our case, no active treatment was given to Mr. A and a complete resolution of symptoms was achieved through supportive care.
Given the dearth of literature regarding EBV transmission via respiratory droplets, a major strength of our case report is that it appears to be the first reported incident of EBV transmission to a medical practitioner following exposure to respiratory droplets from a patient’s sternutation. Another strength is that Mr. A fulfilled a majority of Hoagland’s criteria (bar atypical lymphocyte counts) which we were then able to confirm and monitor the EBV diagnosis through follow up. Unfortunately, it is difficult to always ensure adequate follow up in all patients, and given the atypical presentation of EBV, certain aspects of Hoagland’s criteria in other patients may be absent, even though there is specific antibody evidence of acute EBV infection.
There is a high variance in disease presentation of EBV and a thorough history and examination will facilitate its diagnosis. Given the unpredictable facets concerning EBV transmission and the possibility of severe EBV related complications such as malignancies, the use of masks and practising appropriate hand hygiene are highly recommended.
Acknowledgments
David Hugo Romero for his contributions with the graphic design.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aoi-21-5
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoi-21-5). JHA is the patient in the case (self-authored case). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lennon P, Crotty M, Fenton JE. Infectious mononucleosis. BMJ 2015;350:h1825. [Crossref] [PubMed]
- Sawant SP. Hoagland Sign: An early manifestation of acute infectious mononucleosis- A case report. Curr Pediatr Res 2017;21:400-2.
- Dunmire SK, Hogquist KA, Balfour HH. Infectious Mononucleosis. Curr Top Microbiol Immunol 2015;390:211-40. [Crossref] [PubMed]
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10:321-2. [Crossref] [PubMed]
- Shapiro RS, McClain K, Frizzera G, et al. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 1988;71:1234-43. [Crossref] [PubMed]
- United States Center for Disease Control and Prevention. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007). Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/appendix/type-duration-precautions.html
- Hoagland RJ. Infectious mononucleosis. Prim Care 1975;2:295-307. [PubMed]
- Aronson MD, Komaroff AL, Pass TM, et al. Heterophil antibody in adults with sore throat: frequency and clinical presentation. Ann Intern Med 1982;96:505-8. [Crossref] [PubMed]
- Bass MH. Periorbital edema as the initial sign of infectious mononucleosis. J Pediatr 1954;45:204-5. [Crossref] [PubMed]
- Cohen J. Epstein-Barr virus-infections, including infectious mononucleosis. In: Jameson J, Fauci AS, Kasper DL, et al. editors. Harrison’s Principles of Internal Medicine. 17th edition. US: Mc Graw Hill Med Pub, 2008:1106-8.
- Chervenick PA. Infectious mononucleosis. Dis Mon 1974;1-29. [PubMed]
- Seitanidis B. A comparison of the Monospot with the Paul-Bunnell test in infectious mononucleosis and other diseases. J Clin Pathol 1969;22:321-3. [Crossref] [PubMed]
- Linderholm M, Boman J, Juto P, et al. Comparative evaluation of nine kits for rapid diagnosis of infectious mononucleosis and Epstein-Barr virus-specific serology. J Clin Microbiol 1994;32:259-61. [Crossref] [PubMed]
- Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician 2004;70:1279-87. [PubMed]
- Tetrault G. Infections in heterophile-negative patients. Arch Pathol Lab Med 2001;125:858-9. [Crossref] [PubMed]
- Bruu AL, Hjetland R, Holter E, et al. Evaluation of 12 commercial tests for detection of Epstein-Barr virus-specific and heterophile antibodies. Clin Diagn Lab Immunol 2000;7:451-6. [Crossref] [PubMed]
- van Hasselt W, Schreuder RM, Houwerzijl EJ. Periorbital oedema. Neth J Med 2009;67:338-9. [PubMed]
- Ainsworth C. Building a better lymphoma vaccine. Nature 2018;563:S52-4. [Crossref] [PubMed]
- Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis 1999;31:543-7. [Crossref] [PubMed]
- Candy B, Hotopf M. Steroids for symptom control in infectious mononucleosis. Cochrane Database Syst Rev 2006;(3):CD004402. Update in Cochrane Database Syst Rev 2015;11:CD004402
Cite this article as: Abasszade JH, Tran J, Rama Raj P, Mahdi AA. Atypical transmission of Epstein-Barr virus to a medical practitioner—a case report. Ann Infect 2021;5:4.


