
Staphylococcus aureus transmission in the intensive care unit: the potential role of the healthcare worker carriage
Introduction
Healthcare-associated infection is associated with high morbidity and mortality.
Staphylococcus aureus (S. aureus) is a major agent of healthcare-associated infections and causes a wide range of diseases from mild to life-threatening conditions. Carriage of S. aureus usually precedes infection. The transmission from carrier to non-carrier is therefore at great concern in intensive care units (ICUs). Furthermore, patients in ICUs are exposed to an increased antibiotic selective pressure favoring cross-transmission of resistant bacteria such as methicillin-resistant S. aureus (MRSA) (1,2).
S. aureus colonization
Patients are identified as carriers or non-carriers, and the former are generally classified as “persistent” or “intermittent”. Approximately one fifth of the individuals are persistent carriers in the community (3) and also at ICU admission (4). The main habitat for S. aureus is the anterior nares. Most individuals will be exposed to the organism transiently throughout their lifetime. It was shown that intermittent and non-carriers shared similar nasal elimination kinetics; thus, two types of nasal carriers might be recognized: persistent carriers and “others” (5).
Persistent carriers usually have a higher load and are frequently colonized by a single strain of S. aureus, whereas intermittent carriers may carry different strains over time (6,7).
Furthermore, S. aureus colonization depends on several factors such as genetic or environmental factors, host immunity and bacterial interference (3). Surprisingly, persistent carriers who are artificially colonized with a mixed culture will specifically re-acquire their autologous strain (5). Finally, 80% of strains causing septicemia were endogenous. While the risk of infection in nasal carriers is estimated to be 2- to 12-times higher than in those who are not colonized with S aureus, authors highlighted a lower rate of mortality in persistent carriers compared to others, all possibly explained by a higher rate of anti-staphylococcal antibodies.
Routes of transmission (Figure 1)
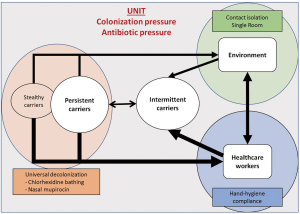
Carriers can be broadly divided into two categories: those already carrying S. aureus at ICU admission, and those with ICU-acquired S. aureus. The first category determines a great part of the colonization pressure. The colonization pressure is the ratio of carriers (colonized or infected) over the number of patients treated in the unit; it remains a major risk factor of S. aureus and MRSA acquisition (8,9). S. aureus acquisition is rarely related to patient-to-patient transmission. It is usually due to transmission through and from healthcare workers (HCWs) or the environment (10). Thus, vectors of transmission largely remain temporarily contaminated hands of the HCWs, either via direct contact with a colonized/infected patient or through simple contact of contaminated surfaces (11). The hand-associated transmission explains why the extensive use of alcohol-based hand-rub solutions for hand disinfection in the 90’s resulted in a sustained reduction of MRSA burden (12).
Studies considering S. aureus acquisition by patients in a room previously occupied by an infected or colonized patient reported contrasting results, suggesting a low risk of direct transmission from the environment, despite the high rate of contaminated surfaces at hospital (10,13).
The risk is maximized if HCW have a sustained carriage (14). For MRSA, carriage among HCWs is favored by chronic skin diseases, poor hygiene practices, and having worked in countries with endemic MRSA (15).
Overall, the proven risk factors associated with a higher risk of MRSA cross-colonization are the colonization pressure, health care workload and absence of single room (16).
Strategies to prevent the transmission by HCWs
Therefore, strategies to limit transmission that consider both patient’s colonization status and risk of transmission from health care worker and the environment were developed.
First, targeted policies are based on active surveillance and contact isolation (the screen-and-isolate strategy). Second, universal policies include universal decolonization and improving hand hygiene. Prior to them, targeted policies were considered as the gold standard. However, recent studies highlighted the effectiveness of universal decolonization including chlorhexidine bathes and nasal mupirocin to prevent MRSA infection outbreak in the ICU (17,18) and may limit the spread of MRSA. Furthermore, it seems to be as effective as contact precaution without the adverse events associated with patient isolation (19,20). It should be noticed that the respective role of improvement of hand hygiene and universal decolonization remains unknown.
Nonetheless, universal decolonization is at risk of alteration of the endogenous human microbiota and may increase the risk of pathogenic infection through a loss of “colonization resistance” (21). Furthermore, this approach presents an inherent risk of emergence of bacterial resistance associated with chlorhexidine and mupirocin use. The potential risk of emergence of resistance to chlorhexidine with the use of universal chlorhexidine bathes is debated in the literature (22-25). Failures of universal decolonization have been linked to chlorhexidine resistance in some recent studies (26,27).
Considering the evidence, it is therefore important to better understand the route of transmission to privilege HCW hand disinfection or universal patient decolonization for interrupting S. aureus transmission.
Whole-genome-sequencing (WGS) and new insight
WGS allows comparison of the genetic difference between organisms and can characterize highly related strains with sufficient resolution to inform on routes of transmission (28). In the context of MRSA outbreak investigation, WGS is useful to identify the source of transmission (14). Moreover, the use of this new technology has revealed that resident type heterogeneity is not exceptional in persistent S. aureus carrier. Polyclonal nasal colonization was identified as well as a colonization by different strains in different niches. Senn et al. even identified a “stealthy colonization of the gut”, revealing a particular clone which prefers digestive tract over the nasal cavity as primary colonization niches. Stealthy carriers probably facilitated the spread of this unrecognized strain of MRSA (29,30).
Prices et al.’s study
Using whole genome sequencing, Price et al. studied those different routes of transmission of S. aureus in ICU and high dependency (31). This longitudinal cohort study collected during 14 months, isolates from 1,854 patients, 198 HCWs and 40 environmental locations. Patients were screened at admission and weekly thereafter whereas HCWs were screened at 4-week intervals.
The rate of S. aureus nasal carriage was 37% for HCWs and 21% for the patients, with 5% and 2% of MRSA respectively. Isolates were defined to be the same subtype if they differed by no more than 40 single-nucleotide variants. Transmissions were defined as an acquisition of a subtype culture from a HCW or the environment either at the same time or at any previous timepoint.
During the period, 97 patient’s acquisition of S. aureus were identified and the transmission source was identified in 25 cases: 7 were from HCWs, 2 from the environment and 16 from other patients. Thus, in 72/97 acquisitions, the source was not identified. The authors concluded that the transmission of S. aureus unlikely came from HCWs.
However, several confounding factors may compromise this finding.
First, an unrecognized HCW intermittent carriage may occur during the 4-week interval between screenings. Of note, in the substudy in which nurses underwent swabbing before and after shifts, no transient carriage was identified.
Second, only two cases of transmission from the environment were identified. Yet, on the 88 strains found in the environment, 51 (57%) were also shared with HCW and/or patients. Transmission from the environment may possibly be underestimated considering the presence of shared rooms.
Third, as only nasal swabs were analyzed, non-nasal or “stealthy” carriage may also explain some unrecognized transmission. However, an overall heterogeneity with multiple different strains was identified during the study without few strains spreading over the ICU. This is in agreement with a cryptic personal carriage of S. aureus which can be developed during the ICU stay.
This study provided very helpful information in the field. However, the external generalizability of the result should be questioned. Indeed, infection control included monthly audits of hand hygiene which may have increased the HCWs compliance with hand hygiene. Furthermore, all patients received skin washes with 4% chlorhexidine solution which may have decreased the patient-to-HCW transmission. Without evaluation of hand-hygiene compliance, no information can be found on which intervention provided this low transmission rate.
Conclusions
In ICU, with a low colonization pressure and good compliance with standard precaution measure, a low rate of HCW-based transmission is highly probable. Thus, additional measures that improve compliance and quality of hand disinfection might be minimally effective to decrease S. aureus cross-transmission and infection.
Future exploration may rely on the transition from colonization to infection. Whole genome sequencing is a valuable tool and could provide information on genetically-related virulence factor. Thereby, the risk of S. aureus infection may be stratified considering the association of genetics features of the bacteria and the personal risk of a patient, allowing targeted intervention (32).
Acknowledgments
We thank Celine Feger (Emibiotech) for her help in writing the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Pu Mao (Associated Researcher of Department of Hospital Infection Management, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoi.2017.08.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weber SG, Gold HS, Hooper DC, et al. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis 2003;9:1415-22. [Crossref] [PubMed]
- Couderc C, Jolivet S, Thiébaut AC, et al. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis 2014;59:206-15. [Crossref] [PubMed]
- Mulcahy ME, McLoughlin RM. Host-Bacterial Crosstalk Determines Staphylococcus aureus Nasal Colonization. Trends Microbiol 2016;24:872-86. [Crossref] [PubMed]
- Porter R, Subramani K, Thomas AN, et al. Nasal carriage of Staphylococcus aureus on admission to intensive care: incidence and prognostic significance. Intensive Care Med 2003;29:655-8. [Crossref] [PubMed]
- van Belkum A, Verkaik NJ, de Vogel CP, et al. Reclassification of Staphylococcus aureus Nasal Carriage Types. J Infect Dis 2009;199:1820-6. [Crossref] [PubMed]
- Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005;5:751-62. [Crossref] [PubMed]
- Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703-5. [Crossref] [PubMed]
- Merrer J, Santoli F, Appéré de Vecchi C, et al. “Colonization pressure” and risk of acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol 2000;21:718-23. [Crossref] [PubMed]
- Bonten MJ. Colonization pressure: a critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit Care 2012;16:142. [PubMed]
- Ajao AO, Harris AD, Roghmann MC, et al. Systematic Review of Measurement and Adjustment for Colonization Pressure in Studies of Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococci, and Clostridium difficile Acquisition. Infect Control Hosp Epidemiol 2011;32:481-9. [Crossref] [PubMed]
- Stiefel U, Cadnum JL, Eckstein BC, et al. Contamination of Hands with Methicillin-Resistant Staphylococcus aureus after Contact with Environmental Surfaces and after Contact with the Skin of Colonized Patients. Infect Control Hosp Epidemiol 2011;32:185-7. [Crossref] [PubMed]
- Jarlier V, Trystram D, Brun-Buisson C, et al. Curbing methicillin-resistant Staphylococcus aureus in 38 French hospitals through a 15-year institutional control program. Arch Intern Med 2010;170:552-9. [Crossref] [PubMed]
- Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections Curr Opin Infect Dis 2013;26:338-44. [Crossref] [PubMed]
- Köser CU, Holden MT, Ellington MJ, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med 2012;366:2267-75. [Crossref] [PubMed]
- Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis 2008;8:289-301. [Crossref] [PubMed]
- Bloemendaal AL, Fluit AC, Jansen WM, et al. Acquisition and Cross-Transmission of Staphylococcus aureus in European Intensive Care Units. Infect Control Hosp Epidemiol 2009;30:117-24. [Crossref] [PubMed]
- Huang SS, Septimus E, Kleinman K, et al. Targeted versus Universal Decolonization to Prevent ICU Infection. N Engl J Med 2013;368:2255-65. [Crossref] [PubMed]
- Derde LP, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis 2014;14:31-9. [Crossref] [PubMed]
- Morgan DJ, Diekema DJ, Sepkowitz K, et al. Adverse outcomes associated with contact precautions: A review of the literature. Am J Infect Control 2009;37:85-93. [Crossref] [PubMed]
- Zahar JR, Garrouste-Orgeas M, Vesin A, et al. Impact of contact isolation for multidrug-resistant organisms on the occurrence of medical errors and adverse events. Intensive Care Med 2013;39:2153-60. [Crossref] [PubMed]
- Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016;352:535-8. [Crossref] [PubMed]
- Batra R, Cooper BS, Whiteley C, et al. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis 2010;50:210-7. [Crossref] [PubMed]
- Hayden MK, Lolans K, Haffenreffer K, et al. Chlorhexidine and Mupirocin Susceptibility of Methicillin-Resistant Staphylococcus aureus Isolates in the REDUCE-MRSA Trial. J Clin Microbiol 2016;54:2735-42. [Crossref] [PubMed]
- Marolf CT, Alter R, Lyden E, et al. Susceptibility of Nosocomial Staphylococcus aureus to Chlorhexidine After Implementation of a Hospital-wide Antiseptic Bathing Regimen. Infect Control Hosp Epidemiol 2017;38:873-5. [Crossref] [PubMed]
- Musuuza JS, Sethi AK, Roberts TJ, et al. Analysis of Multidrug-Resistant Organism Susceptibility to Chlorhexidine Under Usual Clinical Care. Infect Control Hosp Epidemiol 2017;38:729-31. [Crossref] [PubMed]
- Lee AS, Macedo-Vinas M, François P, et al. Impact of combined low-level mupirocin and genotypic chlorhexidine resistance on persistent methicillin-resistant Staphylococcus aureus carriage after decolonization therapy: a case-control study. Clin Infect Dis 2011;52:1422-30. [Crossref] [PubMed]
- Suwantarat N, Carroll KC, Tekle T, et al. Low prevalence of mupirocin resistance among hospital-acquired methicillin-resistant Staphylococcus aureus isolates in a neonatal intensive care unit with an active surveillance cultures and decolonization program. Infect Control Hosp Epidemiol 2015;36:232-4. [Crossref] [PubMed]
- Price JR, Golubchik T, Cole K, et al. Whole-Genome Sequencing Shows That Patient-to-Patient Transmission Rarely Accounts for Acquisition of Staphylococcus aureus in an Intensive Care Unit. Clin Infect Dis 2014;58:609-18. [Crossref] [PubMed]
- Senn L, Clerc O, Zanetti G, et al. The Stealthy Superbug: the Role of Asymptomatic Enteric Carriage in Maintaining a Long-Term Hospital Outbreak of ST228 Methicillin-Resistant Staphylococcus aureus. mBio 2016;7:e02039-15. [Crossref] [PubMed]
- van Belkum A. Hidden Staphylococcus aureus Carriage: Overrated or Underappreciated? mBio 2016;7:e00079-16. [Crossref] [PubMed]
- Price JR, Cole K, Bexley A, et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis 2017;17:207-14. [Crossref] [PubMed]
- Sampedro GR, Bubeck Wardenburg J. Staphylococcus aureus in the Intensive Care Unit: Are These Golden Grapes Ripe for a New Approach? J Infect Dis 2017;215:S64-70. [PubMed]
Cite this article as: Le Bihan C, Zahar JR, Timsit JF. Staphylococcus aureus transmission in the intensive care unit: the potential role of the healthcare worker carriage. Ann Infect 2017;1:3.


